What Phase Is Animal Testing In Clinical Trials
- Clinical research is a branch of healthcare science that performs clinical trials or carries out enquiry experiments on people aiming at finding a solution to a disease.
- Clinical trials are inquiry experiments that determine the condom and effectiveness or efficacy of medications, devices, diagnostic tests, treatments etc. and also monitor and evaluate their effects and outcomes on human health.
- Clinical enquiry is different from clinical practise. In clinical practice, established treatments are used, while in clinical research evidence is collected to establish a treatment.
- Clinical trials determine the efficacy of:
- Drugs
- Vaccines and preventive handling
- Biological products
- Surgical and radiation procedures
- Medical devices
- Cistron therapy etc.
- Major interventions that require homo research experimentation are drug testing and development of vaccine and its testing.
- The initial stages of clinical trial beginning in the laboratories with the production of drugs or vaccines and experimenting on animals and some human cell lines.
- When the first experiment is successful, the review team in the regulating torso known equally Nutrient and Drug Assistants (FDA) reviews and analyzes the data and decides whether to give approval for clinical research and testing straight on humans or not.
- According to the Nutrient and Drug Administration (FDA), a complete clinical trial review squad consists of the following professionals with their corresponding responsibilities:
Clinical trial Review Team:
- Project Director: Coordinates the team's activities throughout the review process, and is the chief contact for the sponsor.
- Medical Officeholder: Reviews all clinical study data and information earlier, during, and later on the trial is complete.
- Microbiologist:Reviews the data submitted, if the production is an antimicrobial product, to appraise response beyond different classes of microbes.
- Statistician:Interprets clinical trial designs and data, and works closely with the medical officer to evaluate protocols and safety and efficacy data.
- Pharmacologist:Reviews pre-clinical studies.
- Pharmacokineticist:Focuses on the assimilation, distribution, and metabolism of drugs, and excretion processes. Interprets blood-level data at different fourth dimension intervals from clinical trials, as a way to assess drug dosages and administration schedules.
- Chemist:Evaluates a drug's chemic compounds. Analyzes how a drug was made and its stability, quality control, continuity, the presence of impurities, etc.
- The testing of the drug or vaccine or medical device can begin one time the FDA approves human experimentation.
- These experiments are washed in iv to five phases where each phase is completely a separate trial although each stage builds on the previous phase.
- Each time a phase trial gets completed, the investigators must nowadays their data for FDA approving before they continue to the next phase.
- The duration of clinical trial can vary based on the type of illness or infection.
- For example, cancer clinical trials are long and can exist done in three phases according to the FDA, while those of novel emerging infections take about several months or at most a year (four to v phases).
Preparation for a clinical trial:
- Similar to a report plan, before any clinical trial, the investigators must prepare a research design, with specific inquiry objectives. The steps that are involved in a clinical trial are known as protocols which accept to be presented to the FDA for approval before beginning the clinical trials This protocols include:
- Choosing the selection criteria of participants who qualify for the report
- The number of participants to involve in the study
- Duration of the study
- Inclusion of a control group and how to limit enquiry bias
- The dosage and elapsing of administrating the drug
- The cess of the inquiry trial should answer what? when? and what information is to be collected
- How to review and clarify the data
Participants in a clinical trial:
- To participate in a clinical trial, a participant must qualify for the study, meeting all the inclusion criteria of age, gender, the type and stage of the disease, previous treatment history, and any other medical weather. The investigator must inform the participant on all possible guidelines (possible risks and outcomes) to participate in the written report.
- People of all age groups including children tin can be involved in a clinical trial once their informed consent is taken.
- Participants can be healthy and sick individuals depending on the type of clinical interventions of investigation and the type of disease or infection. The exclusion and inclusion criteria are meant to identify advisable participants, promote participants' safety, and to ensure the researchers get the required information at the end of the study.
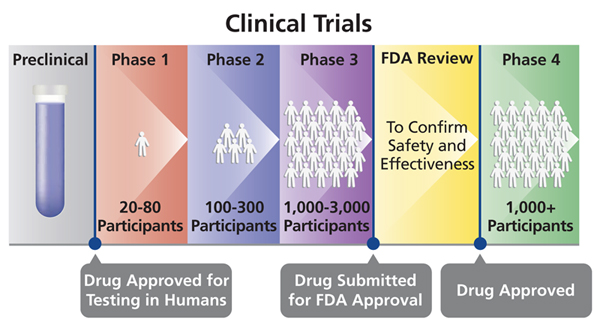
Different phases of Human clinical trials:
- Phase 0
- This is a clinical trial that is washed on a very grouping of people (usually less than 15).
- The investigators volition apply a very small-scale dosage of the medication ensuring that it is harmless before administering a higher dosage for the next phase.
- The outcomes of each private from the experiment are closely monitored, reported and documented accordingly.
- If the individuals react to the medication differently, another preclinical research is washed before making a conclusion on whether to continue or terminate the trial.
- In most trials, Phase 0 is non mandatory and hence investigators movement straight to phase i (I) after preclinical inquiry trials are canonical.
- Phase I
- The investigators motion to Phase I subsequently a positive result outcome of phase 0.
- They utilise a larger group of people (20-80) with no underlying health atmospheric condition. This phase runs upward to several months spending more than time.
- In this phase, a larger dose of drug is used; basically the largest dose a human being being tin can take without serious side effects.
- Shut monitoring of the participants, and continuous reporting and documenting their trunk reactions is done in this phase.
- In this stage, investigators decide the route of the dug administration (intravenous, oral, or topical) based on which can be more effective or depending on the type of infection or disease of investigation.
- Co-ordinate to the FDA, if the medication is from trusted sources and approximately 70% successful, information technology can then move to the next stage, phase Two.
- Phase II
- This phase studies test treatments that have been establish to be safe in phase I but now need a larger grouping of homo subjects to monitor for any adverse effects.
- Information technology involves slightly more than 100 people, who have the status of disease/infection nether investigation.
- The dosage of treatment is the aforementioned as the ane given at stage I. Withal, the population investigated for the trial is notwithstanding not enough for demonstrating the overall safety of the medication but the data collected in this stage broadens the prospects of phase III.
- According to the FDA, about xxx% of the drugs found to exist safe in stage 2 move to Phase III.
- Phase III
- Studies are conducted on even larger populations and in different regions and countries and frequently this is the stage correct earlier a new handling is approved.
- It involves 300- 3000 participants with the status the medication are meant to treat.
- This stage trials can last for a long period (as long as several years) because information technology involves a large group of participants in different regions.
- This stage trials aim at evaluating how the new medication works in comparison to other existing drugs treating the same status, and therefore, the investigators must demonstrate the efficacy, safety, and effectiveness of the medication confronting that of the existing drugs, if any.
- Randomized studies are used whereby participants are randomly selected to grade different sets or groups where one set receives the new medication while another set of participants called controlled placebo groups is administered with an identical merely faux drug normally called sugar pills.
- Phase Three trials are ordinarily double-blind, which means that neither the participant nor the investigator knows which medication the participant is taking. This helps in eliminating bias during issue interpretation.
- Rare and long-term side effects are more likely to bear witness upwardly during this phase because of larger number of participants and longer duration.
- If the investigators can be able to show that the medication is rubber and effective, the FDA can corroborate it for use.
- At to the lowest degree 25-30% of drugs from trusted sources by the FDA move to Phase Iv.
- Stage IV
- In this phase, studies and further testing of drugs in a wide population over a longer time frame is washed, later the drug is approved by a country.
- Investigators use this phase to become more data about the medication's long-term safety, effectiveness, and any other benefits.
Unlike Phases of Clinical Trials (Drugs testing and Evolution of vaccines) in Clinical Enquiry
References:
- books.google.com>Clinical Research – Page 13
- https://www.fda.gov/patients/drug-development-process/step-3-clinical-inquiry
- https://microbenotes.com/phases-of-clinical-trials-for-drugs-and-vaccine-evolution/
Source: https://onlinesciencenotes.com/different-phases-of-clinical-trials-drugs-testing-and-development-of-vaccines-in-clinical-research/
Posted by: sorensontreas1988.blogspot.com

0 Response to "What Phase Is Animal Testing In Clinical Trials"
Post a Comment